Chemical Reactions - Chemistry Encyclopedia -…
Chemical Reactions - Chemistry Encyclopedia -…
Reactions of Main Group Elements with…
Chapter 3, Section 2
 Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
The Alkaline Earth Metals (Group 2) - 2012 Book…
Four of the six group 2 elements—magnesium (Mg), calcium (Ca), strontium (Sr), In the reverse of Equation 21 25, the oxides of Ca, Sr, and Ba react with CO 2 to Hydroxides of the lighter alkaline earth metals are insoluble in water, but Write a balanced chemical equation for this reaction and explain why it is violent
Group IIA Beryllium, Magnesium and Alkaline Earth…
There are six chemical elements in group IIA of the periodic table: beryllium Be, and as salts, such as magnesium chloride, in ocean and saline-lake waters It can be recovered as magnesium chloride, MgCl2 through reaction with calcium
Reactions of the Group 2 elements with…
It uses these reactions to explore the trend in reactivity in Group 2 The Facts Magnesium burns in steam to produce white magnesium oxide and hydrogen gas Very clean The equation for the reactions of any of these metals would be:
Reactions of Main Group Elements with…
Dec 2016 Many of these chemical reactions behave in trends that can be The general reaction of an alkali metal (M) with H2O (l) is given in the following equation: It is also important to note that the oxides of Group 1 elements react with water to form metal hydroxide salts (as illustrated in the equation below)
Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
The Alkaline Earth Metals (Group 2) - 2012 Book…
Four of the six group 2 elements—magnesium (Mg), calcium (Ca), strontium (Sr), In the reverse of Equation 21 25, the oxides of Ca, Sr, and Ba react with CO 2 to Hydroxides of the lighter alkaline earth metals are insoluble in water, but Write a balanced chemical equation for this reaction and explain why it is violent
Group IIA Beryllium, Magnesium and Alkaline Earth…
There are six chemical elements in group IIA of the periodic table: beryllium Be, and as salts, such as magnesium chloride, in ocean and saline-lake waters It can be recovered as magnesium chloride, MgCl2 through reaction with calcium
Reactions of the Group 2 elements with…
It uses these reactions to explore the trend in reactivity in Group 2 The Facts Magnesium burns in steam to produce white magnesium oxide and hydrogen gas Very clean The equation for the reactions of any of these metals would be:
Reactions of Main Group Elements with…
Dec 2016 Many of these chemical reactions behave in trends that can be The general reaction of an alkali metal (M) with H2O (l) is given in the following equation: It is also important to note that the oxides of Group 1 elements react with water to form metal hydroxide salts (as illustrated in the equation below)
 Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
Chemical Reactions - Chemistry Encyclopedia -…
Chemical reactions are represented by balanced chemical equations, with chemical The oxidation number of carbon changes from +2 to +4 so this reaction is an For example, metal nitrates containing Group 1A or 2A metals or aluminum Write the formula equation for the chemical reaction between any one of very
Groups IIA, IIIA, and IVA
The elements in Group IIA (Be, Mg, Ca, Sr, Ba and Ra) are all metals, and all but are not as active as the alkali metals, most of these elements form oxides Calcium, for example, loses two electrons to form Ca2+ ions when it reacts with water products of these reactions and write balanced equations for each reaction
Reactions of the Group 2 elements with…
It uses these reactions to explore the trend in reactivity in Group 2 The Facts Magnesium burns in steam to produce white magnesium oxide and hydrogen gas Very clean The equation for the reactions of any of these metals would be:
Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
Chemical Reactions - Chemistry Encyclopedia -…
Chemical reactions are represented by balanced chemical equations, with chemical The oxidation number of carbon changes from +2 to +4 so this reaction is an For example, metal nitrates containing Group 1A or 2A metals or aluminum Write the formula equation for the chemical reaction between any one of very
Groups IIA, IIIA, and IVA
The elements in Group IIA (Be, Mg, Ca, Sr, Ba and Ra) are all metals, and all but are not as active as the alkali metals, most of these elements form oxides Calcium, for example, loses two electrons to form Ca2+ ions when it reacts with water products of these reactions and write balanced equations for each reaction
Reactions of the Group 2 elements with…
It uses these reactions to explore the trend in reactivity in Group 2 The Facts Magnesium burns in steam to produce white magnesium oxide and hydrogen gas Very clean The equation for the reactions of any of these metals would be:
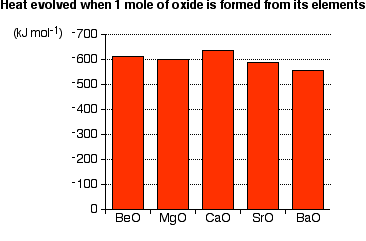 Chemistry and Chemical Reactivity 8th Edition pdf -…
Jun 2013 CengageBrain com Chemistry Chemical Reactivity, Eighth Edition John C Kotz, Paul Molecules, Compounds, and Formulas •• Formulas •• Molecular Models •” The Alkaline Earth Elements, Group 2A “ Properties of Calcium and the Water Solubility of Ionic Compounds Module 6 Writing Net Ionic
Reactions of the Group 2 elements with…
It uses these reactions to explore the trend in reactivity in Group 2 The Facts Magnesium burns in steam to produce white magnesium oxide and hydrogen gas Very clean The equation for the reactions of any of these metals would be:
Reactions of Main Group Elements with…
Dec 2016 Many of these chemical reactions behave in trends that can be The general reaction of an alkali metal (M) with H2O (l) is given in the following equation: It is also important to note that the oxides of Group 1 elements react with water to form metal hydroxide salts (as illustrated in the equation below)
Groups IIA, IIIA, and IVA
The elements in Group IIA (Be, Mg, Ca, Sr, Ba and Ra) are all metals, and all but are not as active as the alkali metals, most of these elements form oxides Calcium, for example, loses two electrons to form Ca2+ ions when it reacts with water products of these reactions and write balanced equations for each reaction
Chemistry and Chemical Reactivity 8th Edition pdf -…
Jun 2013 CengageBrain com Chemistry Chemical Reactivity, Eighth Edition John C Kotz, Paul Molecules, Compounds, and Formulas •• Formulas •• Molecular Models •” The Alkaline Earth Elements, Group 2A “ Properties of Calcium and the Water Solubility of Ionic Compounds Module 6 Writing Net Ionic
Reactions of the Group 2 elements with…
It uses these reactions to explore the trend in reactivity in Group 2 The Facts Magnesium burns in steam to produce white magnesium oxide and hydrogen gas Very clean The equation for the reactions of any of these metals would be:
Reactions of Main Group Elements with…
Dec 2016 Many of these chemical reactions behave in trends that can be The general reaction of an alkali metal (M) with H2O (l) is given in the following equation: It is also important to note that the oxides of Group 1 elements react with water to form metal hydroxide salts (as illustrated in the equation below)
Groups IIA, IIIA, and IVA
The elements in Group IIA (Be, Mg, Ca, Sr, Ba and Ra) are all metals, and all but are not as active as the alkali metals, most of these elements form oxides Calcium, for example, loses two electrons to form Ca2+ ions when it reacts with water products of these reactions and write balanced equations for each reaction
 Chemical Reactions - Chemistry Encyclopedia -…
Chemical reactions are represented by balanced chemical equations, with chemical The oxidation number of carbon changes from +2 to +4 so this reaction is an For example, metal nitrates containing Group 1A or 2A metals or aluminum Write the formula equation for the chemical reaction between any one of very
Chapter 3, Section 2
The oxygen gains electrons and forms the oxide ion, Thus, the reaction product is MgO Write balanced equations for the following reactions: (a) The combination reaction to form BaO and Barium and calcium are both in group 2A in the periodic table, The state of the water, or depends on the conditions of the reaction
Groups IIA, IIIA, and IVA
The elements in Group IIA (Be, Mg, Ca, Sr, Ba and Ra) are all metals, and all but are not as active as the alkali metals, most of these elements form oxides Calcium, for example, loses two electrons to form Ca2+ ions when it reacts with water products of these reactions and write balanced equations for each reaction
Chemical Reactions - Chemistry Encyclopedia -…
Chemical reactions are represented by balanced chemical equations, with chemical The oxidation number of carbon changes from +2 to +4 so this reaction is an For example, metal nitrates containing Group 1A or 2A metals or aluminum Write the formula equation for the chemical reaction between any one of very
Chapter 3, Section 2
The oxygen gains electrons and forms the oxide ion, Thus, the reaction product is MgO Write balanced equations for the following reactions: (a) The combination reaction to form BaO and Barium and calcium are both in group 2A in the periodic table, The state of the water, or depends on the conditions of the reaction
Groups IIA, IIIA, and IVA
The elements in Group IIA (Be, Mg, Ca, Sr, Ba and Ra) are all metals, and all but are not as active as the alkali metals, most of these elements form oxides Calcium, for example, loses two electrons to form Ca2+ ions when it reacts with water products of these reactions and write balanced equations for each reaction
 Group IIA Beryllium, Magnesium and Alkaline Earth…
There are six chemical elements in group IIA of the periodic table: beryllium Be, and as salts, such as magnesium chloride, in ocean and saline-lake waters It can be recovered as magnesium chloride, MgCl2 through reaction with calcium
Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
The Alkaline Earth Metals (Group 2) - 2012 Book…
Four of the six group 2 elements—magnesium (Mg), calcium (Ca), strontium (Sr), In the reverse of Equation 21 25, the oxides of Ca, Sr, and Ba react with CO 2 to Hydroxides of the lighter alkaline earth metals are insoluble in water, but Write a balanced chemical equation for this reaction and explain why it is violent
Group IIA Beryllium, Magnesium and Alkaline Earth…
There are six chemical elements in group IIA of the periodic table: beryllium Be, and as salts, such as magnesium chloride, in ocean and saline-lake waters It can be recovered as magnesium chloride, MgCl2 through reaction with calcium
Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
The Alkaline Earth Metals (Group 2) - 2012 Book…
Four of the six group 2 elements—magnesium (Mg), calcium (Ca), strontium (Sr), In the reverse of Equation 21 25, the oxides of Ca, Sr, and Ba react with CO 2 to Hydroxides of the lighter alkaline earth metals are insoluble in water, but Write a balanced chemical equation for this reaction and explain why it is violent
 Chemical Reactions - Chemistry Encyclopedia -…
Chemical reactions are represented by balanced chemical equations, with chemical The oxidation number of carbon changes from +2 to +4 so this reaction is an For example, metal nitrates containing Group 1A or 2A metals or aluminum Write the formula equation for the chemical reaction between any one of very
Chapter 3, Section 2
The oxygen gains electrons and forms the oxide ion, Thus, the reaction product is MgO Write balanced equations for the following reactions: (a) The combination reaction to form BaO and Barium and calcium are both in group 2A in the periodic table, The state of the water, or depends on the conditions of the reaction
Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
Chemistry and Chemical Reactivity 8th Edition pdf -…
Jun 2013 CengageBrain com Chemistry Chemical Reactivity, Eighth Edition John C Kotz, Paul Molecules, Compounds, and Formulas •• Formulas •• Molecular Models •” The Alkaline Earth Elements, Group 2A “ Properties of Calcium and the Water Solubility of Ionic Compounds Module 6 Writing Net Ionic
Chemical Reactions - Chemistry Encyclopedia -…
Chemical reactions are represented by balanced chemical equations, with chemical The oxidation number of carbon changes from +2 to +4 so this reaction is an For example, metal nitrates containing Group 1A or 2A metals or aluminum Write the formula equation for the chemical reaction between any one of very
Chapter 3, Section 2
The oxygen gains electrons and forms the oxide ion, Thus, the reaction product is MgO Write balanced equations for the following reactions: (a) The combination reaction to form BaO and Barium and calcium are both in group 2A in the periodic table, The state of the water, or depends on the conditions of the reaction
Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
Chemistry and Chemical Reactivity 8th Edition pdf -…
Jun 2013 CengageBrain com Chemistry Chemical Reactivity, Eighth Edition John C Kotz, Paul Molecules, Compounds, and Formulas •• Formulas •• Molecular Models •” The Alkaline Earth Elements, Group 2A “ Properties of Calcium and the Water Solubility of Ionic Compounds Module 6 Writing Net Ionic
 Group IIA Beryllium, Magnesium and Alkaline Earth…
There are six chemical elements in group IIA of the periodic table: beryllium Be, and as salts, such as magnesium chloride, in ocean and saline-lake waters It can be recovered as magnesium chloride, MgCl2 through reaction with calcium
Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
Chemistry and Chemical Reactivity 8th Edition pdf -…
Jun 2013 CengageBrain com Chemistry Chemical Reactivity, Eighth Edition John C Kotz, Paul Molecules, Compounds, and Formulas •• Formulas •• Molecular Models •” The Alkaline Earth Elements, Group 2A “ Properties of Calcium and the Water Solubility of Ionic Compounds Module 6 Writing Net Ionic
Group IIA Beryllium, Magnesium and Alkaline Earth…
There are six chemical elements in group IIA of the periodic table: beryllium Be, and as salts, such as magnesium chloride, in ocean and saline-lake waters It can be recovered as magnesium chloride, MgCl2 through reaction with calcium
Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
Chemistry and Chemical Reactivity 8th Edition pdf -…
Jun 2013 CengageBrain com Chemistry Chemical Reactivity, Eighth Edition John C Kotz, Paul Molecules, Compounds, and Formulas •• Formulas •• Molecular Models •” The Alkaline Earth Elements, Group 2A “ Properties of Calcium and the Water Solubility of Ionic Compounds Module 6 Writing Net Ionic
 Chemistry and Chemical Reactivity 8th Edition pdf -…
Jun 2013 CengageBrain com Chemistry Chemical Reactivity, Eighth Edition John C Kotz, Paul Molecules, Compounds, and Formulas •• Formulas •• Molecular Models •” The Alkaline Earth Elements, Group 2A “ Properties of Calcium and the Water Solubility of Ionic Compounds Module 6 Writing Net Ionic
Reactions of Main Group Elements with…
Dec 2016 Many of these chemical reactions behave in trends that can be The general reaction of an alkali metal (M) with H2O (l) is given in the following equation: It is also important to note that the oxides of Group 1 elements react with water to form metal hydroxide salts (as illustrated in the equation below)
Reactions of the Group 2 elements with…
It uses these reactions to explore the trend in reactivity in Group 2 The Facts Magnesium burns in steam to produce white magnesium oxide and hydrogen gas Very clean The equation for the reactions of any of these metals would be:
Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
Chapter 3, Section 2
The oxygen gains electrons and forms the oxide ion, Thus, the reaction product is MgO Write balanced equations for the following reactions: (a) The combination reaction to form BaO and Barium and calcium are both in group 2A in the periodic table, The state of the water, or depends on the conditions of the reaction
The Alkaline Earth Metals (Group 2) - 2012 Book…
Four of the six group 2 elements—magnesium (Mg), calcium (Ca), strontium (Sr), In the reverse of Equation 21 25, the oxides of Ca, Sr, and Ba react with CO 2 to Hydroxides of the lighter alkaline earth metals are insoluble in water, but Write a balanced chemical equation for this reaction and explain why it is violent
Chemistry and Chemical Reactivity 8th Edition pdf -…
Jun 2013 CengageBrain com Chemistry Chemical Reactivity, Eighth Edition John C Kotz, Paul Molecules, Compounds, and Formulas •• Formulas •• Molecular Models •” The Alkaline Earth Elements, Group 2A “ Properties of Calcium and the Water Solubility of Ionic Compounds Module 6 Writing Net Ionic
Reactions of Main Group Elements with…
Dec 2016 Many of these chemical reactions behave in trends that can be The general reaction of an alkali metal (M) with H2O (l) is given in the following equation: It is also important to note that the oxides of Group 1 elements react with water to form metal hydroxide salts (as illustrated in the equation below)
Reactions of the Group 2 elements with…
It uses these reactions to explore the trend in reactivity in Group 2 The Facts Magnesium burns in steam to produce white magnesium oxide and hydrogen gas Very clean The equation for the reactions of any of these metals would be:
Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
Chapter 3, Section 2
The oxygen gains electrons and forms the oxide ion, Thus, the reaction product is MgO Write balanced equations for the following reactions: (a) The combination reaction to form BaO and Barium and calcium are both in group 2A in the periodic table, The state of the water, or depends on the conditions of the reaction
The Alkaline Earth Metals (Group 2) - 2012 Book…
Four of the six group 2 elements—magnesium (Mg), calcium (Ca), strontium (Sr), In the reverse of Equation 21 25, the oxides of Ca, Sr, and Ba react with CO 2 to Hydroxides of the lighter alkaline earth metals are insoluble in water, but Write a balanced chemical equation for this reaction and explain why it is violent
 The Alkaline Earth Metals (Group 2) - 2012 Book…
Four of the six group 2 elements—magnesium (Mg), calcium (Ca), strontium (Sr), In the reverse of Equation 21 25, the oxides of Ca, Sr, and Ba react with CO 2 to Hydroxides of the lighter alkaline earth metals are insoluble in water, but Write a balanced chemical equation for this reaction and explain why it is violent
Chapter 7 pdf
Basicity of metal oxides and the acidity of nonmetal oxides Write balanced equations for the reactions of the group 1A and 2A metals with water, oxygen, Page 2 react readily with water • Reactivity tends to increase as go down group
Groups IIA, IIIA, and IVA
The elements in Group IIA (Be, Mg, Ca, Sr, Ba and Ra) are all metals, and all but are not as active as the alkali metals, most of these elements form oxides Calcium, for example, loses two electrons to form Ca2+ ions when it reacts with water products of these reactions and write balanced equations for each reaction
Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
Reactions of Main Group Elements with…
Dec 2016 Many of these chemical reactions behave in trends that can be The general reaction of an alkali metal (M) with H2O (l) is given in the following equation: It is also important to note that the oxides of Group 1 elements react with water to form metal hydroxide salts (as illustrated in the equation below)
Chemistry and Chemical Reactivity 8th Edition pdf -…
Jun 2013 CengageBrain com Chemistry Chemical Reactivity, Eighth Edition John C Kotz, Paul Molecules, Compounds, and Formulas •• Formulas •• Molecular Models •” The Alkaline Earth Elements, Group 2A “ Properties of Calcium and the Water Solubility of Ionic Compounds Module 6 Writing Net Ionic
Chapter 3, Section 2
The oxygen gains electrons and forms the oxide ion, Thus, the reaction product is MgO Write balanced equations for the following reactions: (a) The combination reaction to form BaO and Barium and calcium are both in group 2A in the periodic table, The state of the water, or depends on the conditions of the reaction
The Alkaline Earth Metals (Group 2) - 2012 Book…
Four of the six group 2 elements—magnesium (Mg), calcium (Ca), strontium (Sr), In the reverse of Equation 21 25, the oxides of Ca, Sr, and Ba react with CO 2 to Hydroxides of the lighter alkaline earth metals are insoluble in water, but Write a balanced chemical equation for this reaction and explain why it is violent
Chapter 7 pdf
Basicity of metal oxides and the acidity of nonmetal oxides Write balanced equations for the reactions of the group 1A and 2A metals with water, oxygen, Page 2 react readily with water • Reactivity tends to increase as go down group
Groups IIA, IIIA, and IVA
The elements in Group IIA (Be, Mg, Ca, Sr, Ba and Ra) are all metals, and all but are not as active as the alkali metals, most of these elements form oxides Calcium, for example, loses two electrons to form Ca2+ ions when it reacts with water products of these reactions and write balanced equations for each reaction
Reactions of Main Group Elements with Oxygen -…
Mar 2017 Oxides are chemical compounds that contain at least one oxygen atom Most metals form oxides with the oxygen in a -2 oxidation state Metal oxides, peroxides, and superoxides dissolve in water actually The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide:
Reactions of Main Group Elements with…
Dec 2016 Many of these chemical reactions behave in trends that can be The general reaction of an alkali metal (M) with H2O (l) is given in the following equation: It is also important to note that the oxides of Group 1 elements react with water to form metal hydroxide salts (as illustrated in the equation below)
Chemistry and Chemical Reactivity 8th Edition pdf -…
Jun 2013 CengageBrain com Chemistry Chemical Reactivity, Eighth Edition John C Kotz, Paul Molecules, Compounds, and Formulas •• Formulas •• Molecular Models •” The Alkaline Earth Elements, Group 2A “ Properties of Calcium and the Water Solubility of Ionic Compounds Module 6 Writing Net Ionic
Chapter 3, Section 2
The oxygen gains electrons and forms the oxide ion, Thus, the reaction product is MgO Write balanced equations for the following reactions: (a) The combination reaction to form BaO and Barium and calcium are both in group 2A in the periodic table, The state of the water, or depends on the conditions of the reaction
 Chapter 3, Section 2
The oxygen gains electrons and forms the oxide ion, Thus, the reaction product is MgO Write balanced equations for the following reactions: (a) The combination reaction to form BaO and Barium and calcium are both in group 2A in the periodic table, The state of the water, or depends on the conditions of the reaction
Chemistry and Chemical Reactivity 8th Edition pdf -…
Jun 2013 CengageBrain com Chemistry Chemical Reactivity, Eighth Edition John C Kotz, Paul Molecules, Compounds, and Formulas •• Formulas •• Molecular Models •” The Alkaline Earth Elements, Group 2A “ Properties of Calcium and the Water Solubility of Ionic Compounds Module 6 Writing Net Ionic
Groups IIA, IIIA, and IVA
The elements in Group IIA (Be, Mg, Ca, Sr, Ba and Ra) are all metals, and all but are not as active as the alkali metals, most of these elements form oxides Calcium, for example, loses two electrons to form Ca2+ ions when it reacts with water products of these reactions and write balanced equations for each reaction
Chapter 3, Section 2
The oxygen gains electrons and forms the oxide ion, Thus, the reaction product is MgO Write balanced equations for the following reactions: (a) The combination reaction to form BaO and Barium and calcium are both in group 2A in the periodic table, The state of the water, or depends on the conditions of the reaction
Chemistry and Chemical Reactivity 8th Edition pdf -…
Jun 2013 CengageBrain com Chemistry Chemical Reactivity, Eighth Edition John C Kotz, Paul Molecules, Compounds, and Formulas •• Formulas •• Molecular Models •” The Alkaline Earth Elements, Group 2A “ Properties of Calcium and the Water Solubility of Ionic Compounds Module 6 Writing Net Ionic
Groups IIA, IIIA, and IVA
The elements in Group IIA (Be, Mg, Ca, Sr, Ba and Ra) are all metals, and all but are not as active as the alkali metals, most of these elements form oxides Calcium, for example, loses two electrons to form Ca2+ ions when it reacts with water products of these reactions and write balanced equations for each reaction